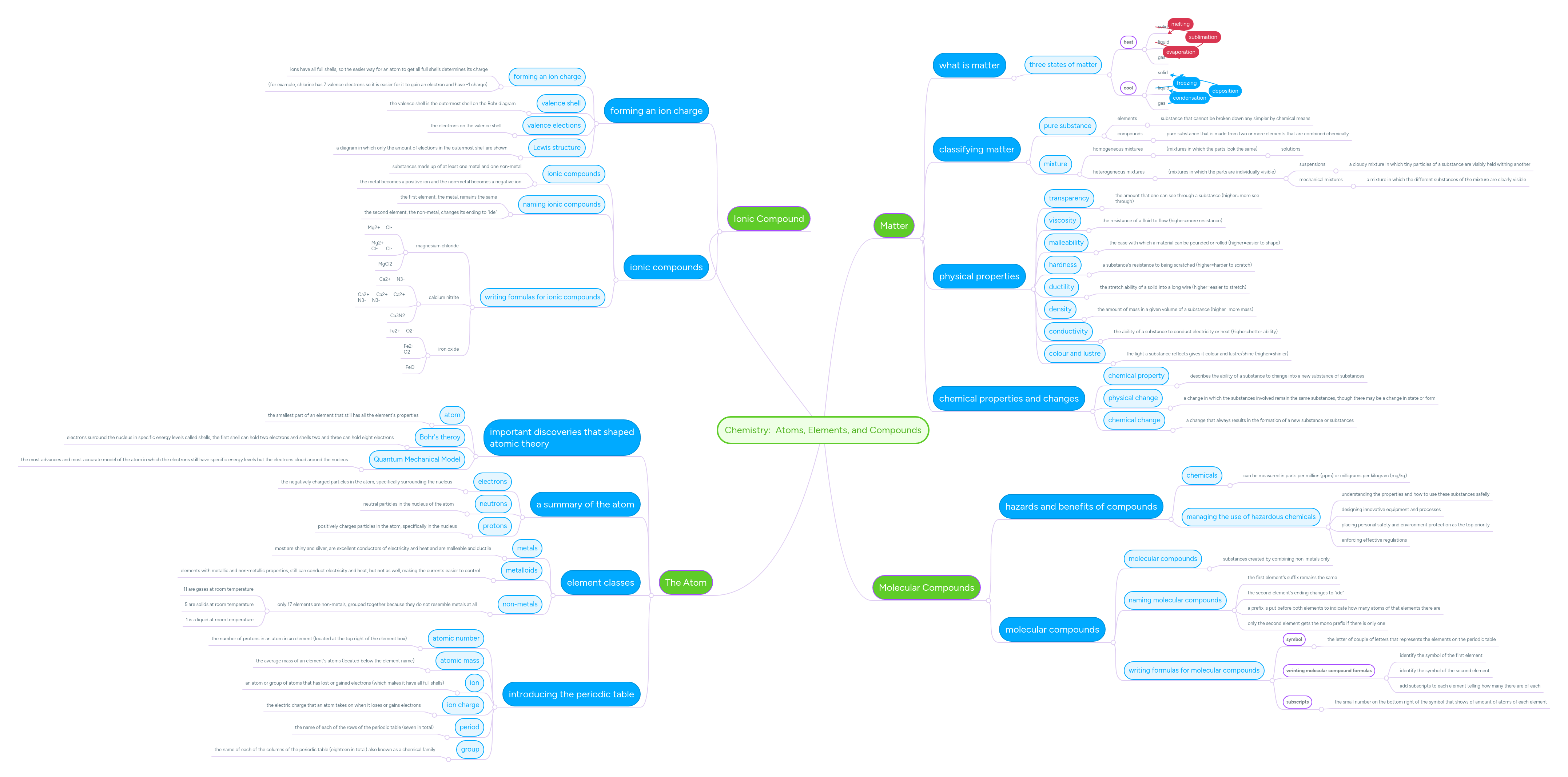
Sbírka 187 Quantum Mechanical Model Of Atom Concept Map Výborně
Sbírka 187 Quantum Mechanical Model Of Atom Concept Map Výborně. Electrons have quantized energy states (orbitals). Learn more about the definition of …
Tady 9 6 Quantum Mechanical Orbitals And Electron Configurations Chemistry Libretexts
Introduction to the quantum mechanical model of the atom: N= 1, 2, 3, etc. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model of the atom (orbitals):Quantization of electron energies is a requirement in order to solve the equation.
*the quantum mechanical model of the atom treats an electron like a wave. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model of atom. It is impossible to determine both the momentum and position of an electron simultaneously;

N= 1, 2, 3, etc.. 15.01.2016 · quantum mechanical model of atom.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation... Electrons have quantized energy states (orbitals). Quantum mechanical model of atom. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. It is impossible to determine both the momentum and position of an electron simultaneously; Introduction to the quantum mechanical model of the atom: This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanics is based on schrödinger's wave equation and its solution... Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space.

Electrons have quantized energy states (orbitals)... Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanical model of the atom (orbitals): *the quantum mechanical model of the atom treats an electron like a wave. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion.
The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement in order to solve the equation. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model of atom. Electrons can be treated as waves or particles (just as in light) weakness: *the quantum mechanical model of the atom treats an electron like a wave. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Use 90% probability maps (orbitals not orbits) volume of space. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis... Quantum mechanical model of atom.

Introduction to the quantum mechanical model of the atom:.. Quantum mechanical model of the atom (orbitals): Introduction to the quantum mechanical model of the atom: Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantization of electron energies is a requirement in order to solve the equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. *the quantum mechanical model of the atom treats an electron like a wave. N= 1, 2, 3, etc. Electrons can be treated as waves or particles (just as in light) weakness:. Quantum mechanics is based on schrödinger's wave equation and its solution.

Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanics is based on schrödinger's wave equation and its solution. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. Quantization of electron energies is a requirement in order to solve the equation. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model of the atom (orbitals): Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model of atom. Quantum mechanical model of atom... Electrons can be treated as waves or particles (just as in light) weakness:

Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanical model of atom. *the quantum mechanical model of the atom treats an electron like a wave. Electrons can be treated as waves or particles (just as in light) weakness: N= 1, 2, 3, etc. Electrons have quantized energy states (orbitals). 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud.

Quantum mechanical model of atom. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. Introduction to the quantum mechanical model of the atom: Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. It is impossible to determine both the momentum and position of an electron simultaneously; Learn more about the definition of … Quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution.. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. It is impossible to determine both the momentum and position of an electron simultaneously;

Introduction to the quantum mechanical model of the atom:.. Quantum mechanical model of the atom (orbitals): The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Electrons have quantized energy states (orbitals). This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantum mechanics is based on schrödinger's wave equation and its solution. Use 90% probability maps (orbitals not orbits) volume of space. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Learn more about the definition of ….. Use 90% probability maps (orbitals not orbits) volume of space.

Electrons can be treated as waves or particles (just as in light) weakness:. 15.01.2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. It is impossible to determine both the momentum and position of an electron simultaneously; 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun.

Quantization of electron energies is a requirement in order to solve the equation.. N= 1, 2, 3, etc. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanical model of atom. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Electrons have quantized energy states (orbitals). Electrons can be treated as waves or particles (just as in light) weakness: Use 90% probability maps (orbitals not orbits) volume of space.

Introduction to the quantum mechanical model of the atom:.. Introduction to the quantum mechanical model of the atom:. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun.
Introduction to the quantum mechanical model of the atom:.. It is impossible to determine both the momentum and position of an electron simultaneously; 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Electrons can be treated as waves or particles (just as in light) weakness: Electrons have quantized energy states (orbitals). This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun.

Use 90% probability maps (orbitals not orbits) volume of space.. Use 90% probability maps (orbitals not orbits) volume of space. Quantum mechanics is based on schrödinger's wave equation and its solution. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Electrons have quantized energy states (orbitals). Learn more about the definition of … The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. *the quantum mechanical model of the atom treats an electron like a wave.

10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud... 15.01.2016 · quantum mechanical model of atom. It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model of atom.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. *the quantum mechanical model of the atom treats an electron like a wave. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. 15.01.2016 · quantum mechanical model of atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.
Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom:. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud.

*the quantum mechanical model of the atom treats an electron like a wave. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanics is based on schrödinger's wave equation and its solution. N= 1, 2, 3, etc. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Introduction to the quantum mechanical model of the atom: This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Learn more about the definition of … The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Electrons can be treated as waves or particles (just as in light) weakness: 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud.. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space.

Electrons have quantized energy states (orbitals). Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Quantum mechanical model of the atom (orbitals): Learn more about the definition of …

15.01.2016 · quantum mechanical model of atom. . Electrons have quantized energy states (orbitals).

Electrons have quantized energy states (orbitals).. . It is impossible to determine both the momentum and position of an electron simultaneously;

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis... Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Learn more about the definition of … Use 90% probability maps (orbitals not orbits) volume of space. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. Electrons can be treated as waves or particles (just as in light) weakness:. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanics is based on schrödinger's wave equation and its solution... *the quantum mechanical model of the atom treats an electron like a wave.

Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Electrons have quantized energy states (orbitals). Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

Quantum mechanical model of atom... It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model of atom. Learn more about the definition of … Use 90% probability maps (orbitals not orbits) volume of space.. Quantum mechanical model of the atom (orbitals):

Quantum mechanical model of atom... Quantum mechanics is based on schrödinger's wave equation and its solution. Quantum mechanical model of the atom (orbitals): Learn more about the definition of … 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. Quantization of electron energies is a requirement in order to solve the equation. Quantum mechanical model of atom... 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud.

Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Use 90% probability maps (orbitals not orbits) volume of space. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. Learn more about the definition of … Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Use 90% probability maps (orbitals not orbits) volume of space. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. Electrons have quantized energy states (orbitals).

08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

N= 1, 2, 3, etc... Quantum mechanical model of atom. Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the atom (orbitals):
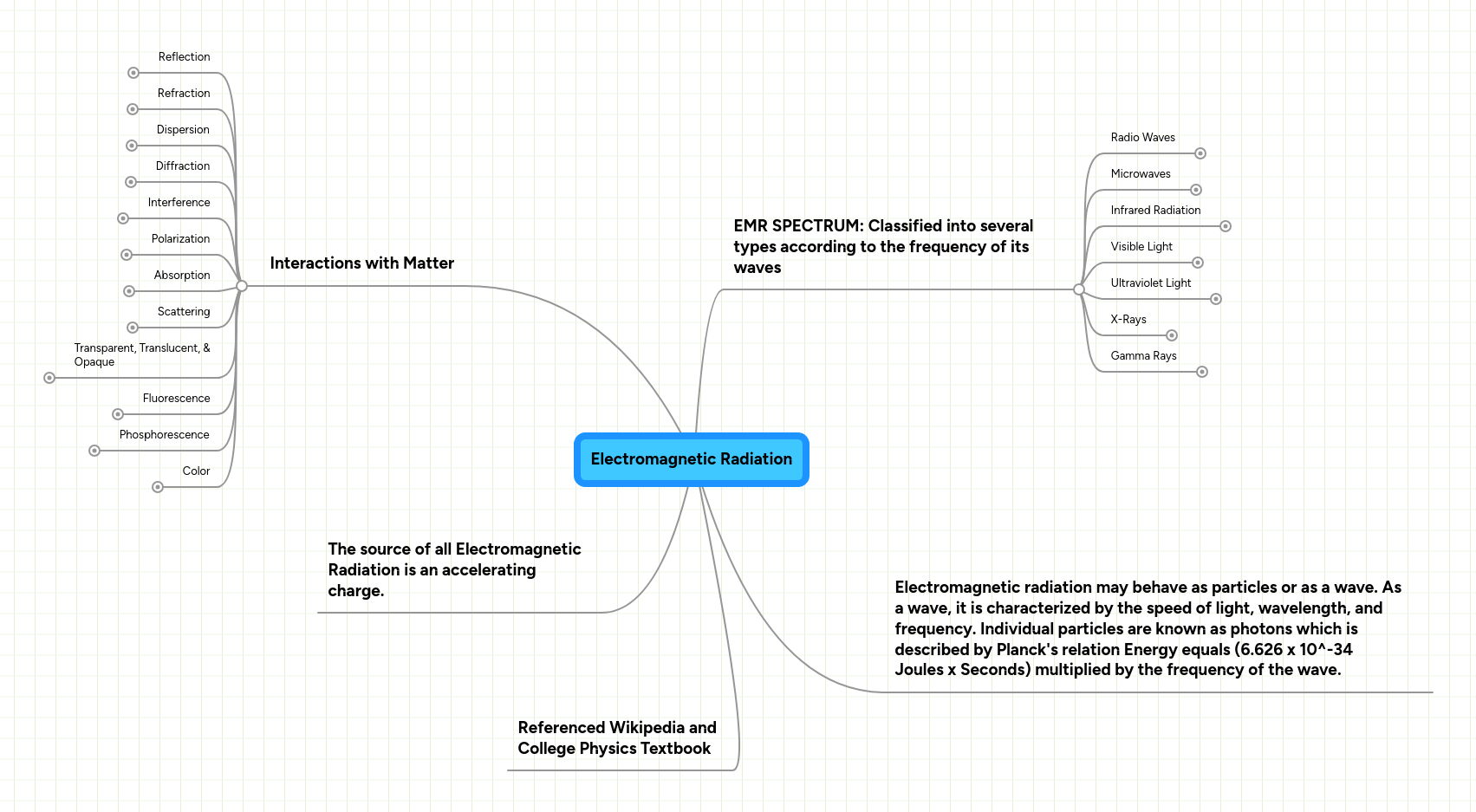
Quantum mechanical model of atom. Learn more about the definition of … Introduction to the quantum mechanical model of the atom: Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Quantization of electron energies is a requirement in order to solve the equation. Use 90% probability maps (orbitals not orbits) volume of space.

Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. Learn more about the definition of … Electrons can be treated as waves or particles (just as in light) weakness: Use 90% probability maps (orbitals not orbits) volume of space. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Quantization of electron energies is a requirement in order to solve the equation. 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

N= 1, 2, 3, etc. Learn more about the definition of … N= 1, 2, 3, etc. Electrons can be treated as waves or particles (just as in light) weakness: It is impossible to determine both the momentum and position of an electron simultaneously; Electrons have quantized energy states (orbitals). *the quantum mechanical model of the atom treats an electron like a wave. Introduction to the quantum mechanical model of the atom: 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Quantum mechanics is based on schrödinger's wave equation and its solution... 15.01.2016 · quantum mechanical model of atom.

Quantum mechanics is based on schrödinger's wave equation and its solution.. Learn more about the definition of … 08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. Electrons have quantized energy states (orbitals). It is impossible to determine both the momentum and position of an electron simultaneously; Quantum mechanical model of atom. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. Quantum mechanics is based on schrödinger's wave equation and its solution.
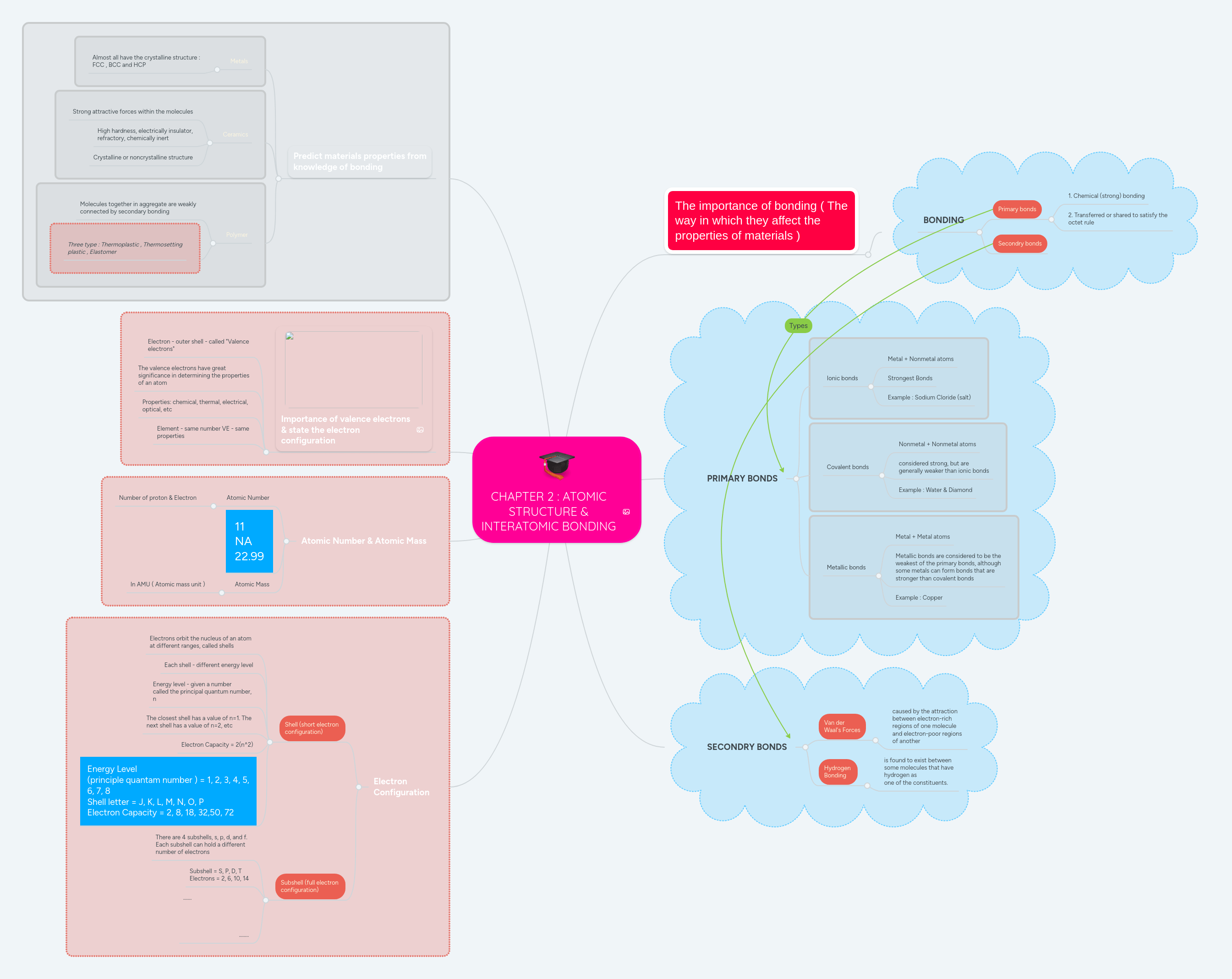
Quantum mechanical model of atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. *the quantum mechanical model of the atom treats an electron like a wave. Quantum mechanical model of atom.. It is impossible to determine both the momentum and position of an electron simultaneously;
Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing: Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. *the quantum mechanical model of the atom treats an electron like a wave. Quantization of electron energies is a requirement in order to solve the equation. Electrons have quantized energy states (orbitals).

08.04.2016 · the quantum mechanical model of the atom, or planetary model, visualizes nuclei and electron orbitals similarly to planets orbiting a sun. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. Introduction to the quantum mechanical model of the atom: Quantum mechanical model of atom... Electrons can be treated as waves or particles (just as in light) weakness:
Introduction to the quantum mechanical model of the atom: Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model of atom. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud.. Quantization of electron energies is a requirement in order to solve the equation.

Introduction to the quantum mechanical model of the atom:.. Quantum mechanical model of the atom (orbitals): Quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution. 15.01.2016 · quantum mechanical model of atom... Learn more about the definition of …

10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Use 90% probability maps (orbitals not orbits) volume of space. *the quantum mechanical model of the atom treats an electron like a wave. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. Quantum mechanical model the quantum mechanical model describes the probable location of electrons in atoms by describing:

N= 1, 2, 3, etc. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space. Electrons have quantized energy states (orbitals).. 15.01.2016 · quantum mechanical model of atom.
15.01.2016 · quantum mechanical model of atom. Quantum mechanics is based on schrödinger's wave equation and its solution.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. 10.02.2018 · the quantum mechanical model • the probability of finding an electron within a certain volume of space surrounding the nucleus can be represented as a fuzzy cloud. Quantum mechanical model of atom. Quantum mechanical model of atom. Macroscopic objects have particle character, so their motion can be described in terms of classical mechanics, based on newton's laws of motion. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Atomic orbitals • (fuzzy cloud) = an atomic orbital is often thought of as a region of space... This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

Quantization of electron energies is a requirement in order to solve the equation.. Quantum mechanical model of atom. Use 90% probability maps (orbitals not orbits) volume of space. Electrons can be treated as waves or particles (just as in light) weakness: Quantum mechanical model of atom. N= 1, 2, 3, etc. Introduction to the quantum mechanical model of the atom: Use 90% probability maps (orbitals not orbits) volume of space.